Introduction: High dose chemotherapy and autologous stem cell transplantation (ASCT) is one of the most effective therapies in light chain (AL) amyloidosis. It is associated with a high hematologic response rate and leads to durable remissions. High dose melphalan is the most frequently used conditioning chemotherapy, as has been used in multiple myeloma (MM). Melphalan dose is based on renal function, age, degree of cardiac involvement and patients' frailty and is predictive of outcomes. While studies that assessed the conditioning schedule have been conducted in MM, with variable results, no such studies were done in AL amyloidosis.
Methods: In this retrospective study, we explored the impact of the conditioning schedule on post-transplant outcomes in a large multicenter study. Patients with AL amyloidosis who underwent ASCT for the primary diagnosis of AL amyloidosis between January 1, 2003, and December 31, 2020, were included in this study.
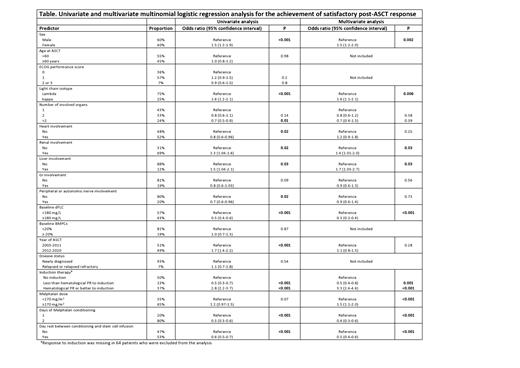
Results: One thousand seven hundred and eighteen (n=1718) patients from 9 centers are included. The median age was 58 years, and 61% were male. Most patients had one (43%) or two (33%) involved organs, with the most involved organs were the kidneys (69%) followed by the heart (52%). Induction therapy was given to 52% of patients. The conditioning regimen was almost exclusively melphalan (99.2%), with 65% receiving full dose melphalan (≥170 mg/m 2). Melphalan was given over two days in most patients (80%). A rest day between conditioning and stem cell infusion was provided in 53% of patients, mostly in those who received melphalan on a single day rather than over 2 days (87% vs 44%, P<0.001). Post-transplant hematologic response was available for 1569 patients (91% of patients) and were assessed from baseline. Complete response (CR) and very good partial responses (VGPR) were achieved post-ASCT in 33.7% of patients each. Low dFLC-partial response (PR) was achieved in 2.7% and PR in 18% of patients. Altogether, satisfactory post-ASCT response (CR, VGPR, or low-dFLC PR combined) was achieved in 70% of patients. Compared to no induction, less than a PR to induction was associated with a lower probability of achieving satisfactory post-ASCT response, while PR or better to induction had higher chances of satisfactory post-ASCT response (65% vs 46% vs 84%, P<0.001). A single day of melphalan conditioning was associated with a higher probability of satisfactory post-ASCT response compared to two days of conditioning (82% vs 67%, P<0.001). Omitting a day of rest between conditioning and stem cell infusion was also associated with higher satisfactory post-ASCT response compared to having a rest day (76% vs 65%, P<0.001). The Table lists other predictors for a post-ASCT satisfactory hematologic response. In a nominal multivariate logistic regression analysis, independent predictors for higher post-ASCT satisfactory hematological response rate included: PR or better to induction therapy, female gender, kappa light chain isotype, lower dFLC at baseline, and kidney and liver involvement. In addition, full dose melphalan, conditioning given over a single day and having no rest day between conditioning and stem cell infusion were also associated with a higher likelihood of satisfactory post-ASCT response. Post-ASCT hematologic CR rate was higher in those receiving full dose conditioning (36% vs 28%, P<0.001), in those having no rest day (40% vs 28%, P<0.001) but not in those who received one day of conditioning versus two days (35% vs 33%, P=0.5). The rate of ICU admission was higher in those receiving no day rest (17.7% vs 12.6%), P=0.003) and among those who received reduced dose melphalan (18.1% vs 13.3%, P=0.008) but not based on number of conditioning days (P=0.56). Day 100 mortality was significantly higher in the no rest day group (5.6% vs 3.2%, P=0.01) and among those receiving reduced dose melphalan (6.8% vs 3.1%, P=0.004) compared to their counterparts, but not based on the number of days of conditioning (P=0.89). The median follow-up in the entire cohort was 104 months (95% CI 98-110). In a multivariate analysis for overall survival (OS), the conditioning dose but not the conditioning schedule was independently associated with OS.
Conclusions: This study is the first to demonstrate the effect of melphalan conditioning schedule on post-transplant response. This study supports a single day melphalan conditioning in AL amyloidosis to improve post-ASCT response.
Disclosures
Muchtar:Protego: Consultancy. Sanchorawala:Janssen, Alexion, Prothena, Celgene, Takeda, Abbvie, Regeneron, Pfizer, AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Hegenbart:Alnylam: Honoraria, Speakers Bureau; Prothena: Honoraria, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Lee:Amgen: Research Funding; Janssen: Consultancy, Research Funding; Genentech: Consultancy; Allogene Therapeutics: Consultancy; Takeda: Consultancy, Research Funding; Pfizer: Consultancy; Sanofi: Consultancy; Bristol Myers Squibb: Consultancy, Research Funding; Regeneron: Consultancy, Research Funding; GlaxoSmithKline: Consultancy, Research Funding. Qazilbash:Amgen: Research Funding; Bioline: Other: Advisory board; NexImmune: Research Funding; Janssen: Research Funding; Angiocrine: Research Funding. Houde:Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Research Funding; Alnylam, Amgen, CaelumBiosciences, Celgene, Intellia,Janssen, Prothena, Regeneron: Consultancy; Alexion, AstraZeneca Rare Diseases: Research Funding; Janssen: Research Funding. Dispenzieri:Janssen: Membership on an entity's Board of Directors or advisory committees; Alnylam, Bristol-Myers Squibb, Janssen, Pfizer, Takeda: Research Funding; Oncopeptides, Sorento: Consultancy. Chakraborty:Adaptive Biotech: Consultancy, Honoraria; Genentech: Research Funding; AbbVie: Research Funding; Sanofi: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Lentzsch:Alexion Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol Meyers Squibb: Membership on an entity's Board of Directors or advisory committees; Sanofi: Research Funding; Caelum Biosciences: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: January 1, 2041; Celgene: Research Funding; Regeneron: Honoraria; Clinical Care Options: Honoraria; Janssen: Membership on an entity's Board of Directors or advisory committees; Karyopharm Therapeutics: Membership on an entity's Board of Directors or advisory committees; Oncopeptide: Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; Adaptive Biotechnologies: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees. Landau:Alexion Pharmaceuticals, Takeda, Janssen, Prothena, Protego: Research Funding; Karyopharm, Pfizer, Juno, Prothena, Caelum Biosiences, Legend Biotech, Takeda, Janssen, Nexcella: Honoraria. Gertz:Sorrento: Honoraria; Sanofi: Honoraria; Prothena: Honoraria; Johnson & Johnson: Honoraria; Janssen: Honoraria; Ionis/Akcea: Honoraria; Celgene: Honoraria; Ashfield: Honoraria, Research Funding; Aptitude: Honoraria; AbbVie: Honoraria; Juno: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal